Bulky triarylarsines are effective ligands for palladium catalysed Heck olefination - Dalton Transactions (RSC Publishing)
podstata implicitní Nebezpečný bulky triarylarsines are effective ligands for palladium catalysed heck salon nikl šrot
Bulky triarylarsines are effective ligands for palladium catalysed Heck olefination - Dalton Transactions (RSC Publishing) DOI:10.1039/B417910B

Bulky triarylarsines are effective ligands for palladium catalysed Heck olefination - Dalton Transactions (RSC Publishing) DOI:10.1039/B417910B

PDF) 📄 Electron-Deficient Phosphines Accelerate the Heck Reaction of Electronrich Olefins in Ionic Liquid
Inorganics | Free Full-Text | Metal–Organic Frameworks as Versatile Platforms for Organometallic Chemistry | HTML
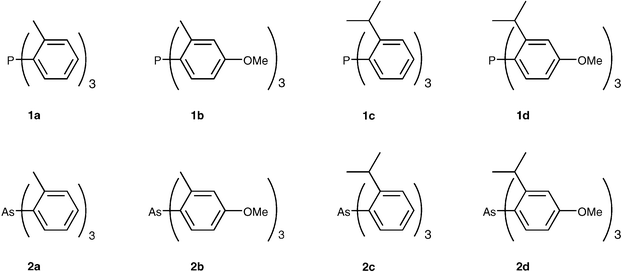
podstata implicitní Nebezpečný bulky triarylarsines are effective ligands for palladium catalysed heck salon nikl šrot
Highly Efficient Palladium-Catalyzed Arsination. Synthesis of a Biphenyl Arsine Ligand and Its Application to Obtain Perfluoroal

Low-coordinate M(0) complexes of group 10 stabilized by phosphorus(III) ligands and N-heterocyclic carbenes - ScienceDirect
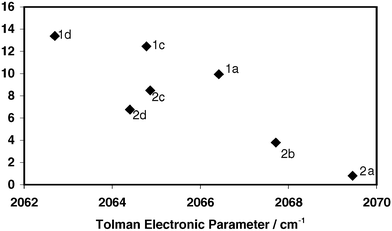
podstata implicitní Nebezpečný bulky triarylarsines are effective ligands for palladium catalysed heck salon nikl šrot
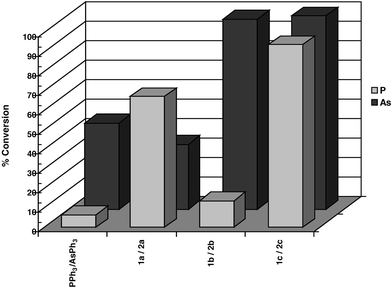
Bulky triarylarsines are effective ligands for palladium catalysed Heck olefination - Dalton Transactions (RSC Publishing) DOI:10.1039/B417910B